Sickle Cell Disease (SCD) is a hereditary disease, and a worldwide public health problem, with a higher incidence in sub-Saharan Africa, where the prevalence is very high (about 80%). SCD prevalence can reach 3,3 % in Angola, while carriers of the sickle cell trait account for more than 25% of the population. The early identification of patients and their follow-up in consultation are an added value for the reduction of morbidity and mortality of children with SCD. The implementation of early infant screening and follow-up programs for children with SCD showed excellent results in increasing the survival of these children. With this project we intend to implement a Newborn screening in Hospital Materno Infantil Dr Manuel Pedro Azancot de Menezes, a referral of the main maternity in Angola, located in Luanda, the country capital, that could be replicated in other hospitals, giving training to the health professionals, and to the laboratory technicians, in order to diagnose the SCD patients.
The objective of this project is to establish a neonatal screening for SCD at a main Hospital in Angola, contributing to reduce the under 5 mortality, and to support for pediatric follow-up consultations of children diagnosed with SCD. Specific aims include: record the data and medical history of babies diagnosed with SCD up to 5 years of age at regular appointments; estimate the prevalence of SCD in the municipality of Camama, Luanda; and to evaluate the costs of neonatal screening and early interventions in Angola, by supporting a registry of SCD.
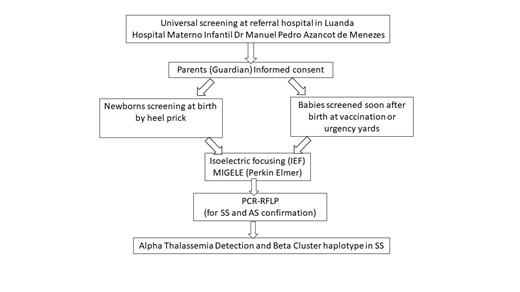
The newborn screening has started in June 2023, with the training of the team at the hospital, and with the creation of all documents and forms needed. The project received ethical approval in June 2023. The study includes every child born in the maternity ward of the Hospital, and also every child that take vaccinations there (in this way we can include newborns delivered at home), that the legal guardians authorize the collection of blood sample by prick heel for screening for SCD. The samples of filter paper duly identified with the sample number and date of collection are being sent to Lisbon (Portugal), for hemoglobin electrophoresis by isoelectric focusing (IEF) carried out in the Migele equipment (acquired with the support of Perkin Elmer, and the ARISE project) (Figure 1).
All SS samples are being genotyped for the HbS mutation by PCR-RFLP. Since the start of the newborn screening (June 28 th) we have collected 1000 samples in 4 weeks and have started the IEF analysis with MIGELE (Perkin Elmer). The preliminary results (221 samples, 177 AA, 41 AS and 3 SS) showed a 1.4% prevalence of SS, and 19.5% of AS, confirmed by PCR-RFLP (in all AS and SS). The frequencies were in Hardy-Weinberg equilibrium. Moreover, we are searching for other possible hemoglobinopathies. More results will be presented. All children diagnosed as SS (SCD) are being contacted by the Hospital Materno Infantil Dr Manuel Pedro Azancot de Menezes, Angola, to be followed in consultation. Newborn screening and early interventions for SCD should be a health priority and implemented at national level. It is our plan to catalyze progress toward this aim by demonstrating the high prevalence of neonatal SCD in the Luanda Hospital and demonstrate the feasibility of this screening in reducing under 5 mortalities.
The present project has the support of ARISE project “African Research and Innovative initiative for Sickle cell Education: Improving Research Capacity for Service Improvement” project (GA 824021 - ARISE - H2020-MSCA-RISE-2018) by supporting the secondments of Angolan Laboratory technicians in Lisbon (Portugal) to perform the IEF and genetic analysis and be trained. Until now 5 Angolan secondees, and 3 Nigerian secondees have been trained in Lisbon. Perkin Elmer (Revvity) is giving llogistical support by kindly support the low-cost acquisition, installation and training in MIGELE equipment for IEF, and being always available to technical support.
Figure 1 - Flow diagram of the methodology followed in the present study.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal